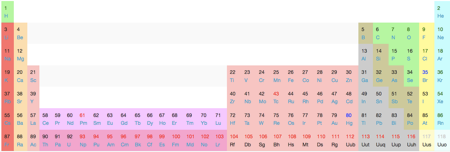
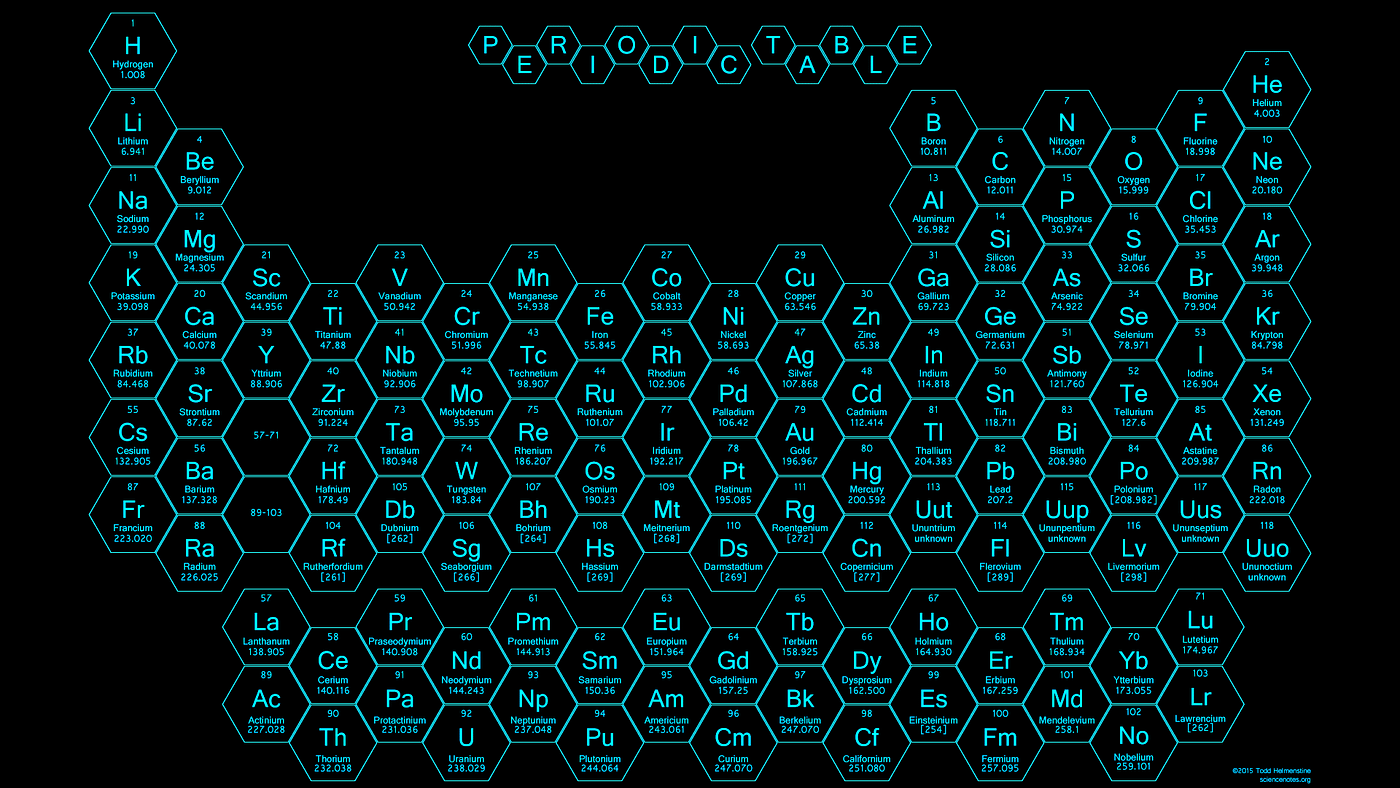
Using correct periodic table terminology we would say these are elements found in groups 1 2 and 13 through 18. The top row of that island makes up the lanthanides and the bottom row makes up the actinides.
Why Does The Periodic Table Have That Separated Part Lanthanides And Actinides At The Bottom By Youssef Zohbi Medium
You may notice that we are leaving out that island of elements down below.

. Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens it exists as a semi-lustrous non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius and boils to a violet gas at 184 degrees CelsiusThe element was discovered by the French chemist Bernard Courtois in 1811 and was named two. The transition metals are found in groups 3 through 12.
Why Does The Periodic Table Have That Separated Part Lanthanides And Actinides At The Bottom By Youssef Zohbi Medium

History Of Chemistry Why Lanthanides And Actinides Are Shown Separate From Standard Periodic Table Layout Chemistry Stack Exchange

Why Are Lanthanides And Actinides Are Kept Out Of The Periodic Table Quora
0 Comments